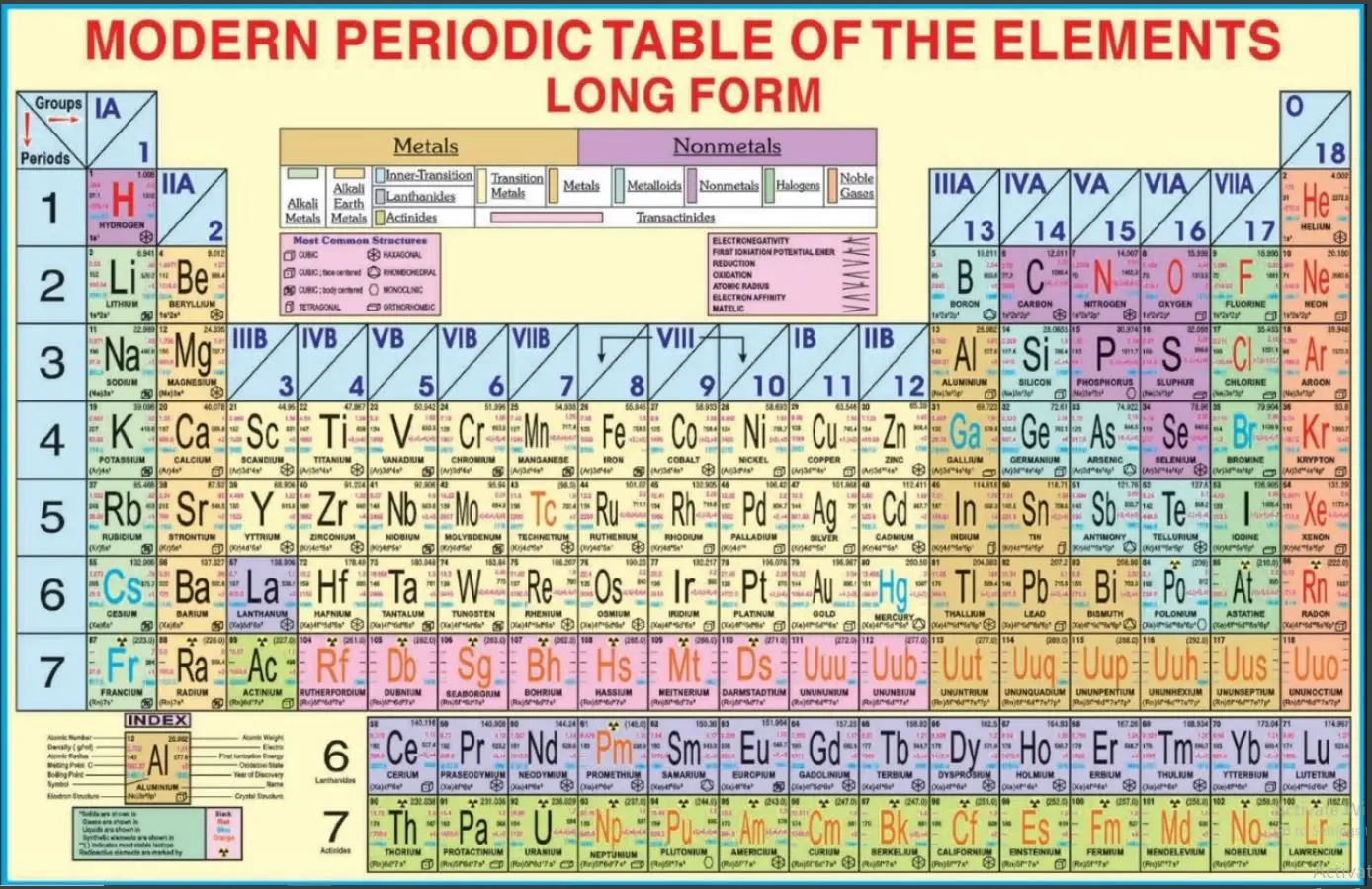
The Periodic Table of Elements is a systematically organized chart that displays all known chemical elements based on their atomic number, electron configurations, and recurring chemical properties. It serves as a foundational tool in chemistry, physics, and materials science.
It Features the below:
-
Element names, symbols, and atomic numbers
-
Atomic weights
-
Color coding by element groups (e.g., alkali metals, noble gases, transition metals)
-
Lanthanides and actinides separately indicated
-
Optional features: electron configurations, oxidation states, or IUPAC updates
Structure and Organization:
-
Rows (Periods): The horizontal rows represent elements with the same number of atomic shells. Moving from left to right across a period, elements show a gradual progression in properties such as electronegativity and ionization energy.
-
Columns (Groups or Families): The vertical columns group elements with similar chemical and physical properties. Elements in the same group have the same number of electrons in their outermost shell, leading to similar reactivity.
-
Blocks: The table is divided into blocks (s, p, d, and f) based on the electron subshell being filled:
-
s-block: Groups 1 and 2
-
p-block: Groups 13 to 18
-
d-block: Transition metals (Groups 3 to 12)
-
f-block: Lanthanides and actinides
-
Categories of Elements:
-
Metals: Found mostly on the left and center; typically malleable, ductile, and good conductors.
-
Nonmetals: Located on the right; they exhibit high electronegativity and low conductivity.
-
Metalloids: Elements with properties intermediate between metals and nonmetals.
-
Noble Gases: Inert gases in Group 18 with full valence electron shells.
-
Alkali and Alkaline Earth Metals: Highly reactive metals in Groups 1 and 2.
-
Transition Metals: Elements with variable oxidation states and complex ion formation.
-
Lanthanides and Actinides: Inner transition metals with specialized applications in technology and nuclear chemistry.
Scientific Significance:
-
Predicts element behavior and trends in atomic size, electronegativity, ionization energy, and chemical reactivity.
-
Enables classification and systematic study of chemical compounds.
-
Forms the basis for modern theoretical models in atomic and molecular chemistry.









Reviews
There are no reviews yet.